So as I mention, we necropsied red eared sliders for this wet lab. Most of the sliders had been part of a research group, and the cause of death was unknown. That wasn't really why we were originally intending to do this, but that's what we ended up doing.
The major difference between necropsying a turtle and any other reptile is that really hard thing called a shell that gets in your way. We had to saw through the shells on all of the turtles to gain access to the rest of the body cavities. And the muscles on a turtle's legs are actually attached to the shell on the inside, so you can't just saw one side open and then lift it up like your opening a lid. It takes a surprising amount of work. In fact, it took us longer to get the shell off than it did to identify the cause of death.
Opening the shell
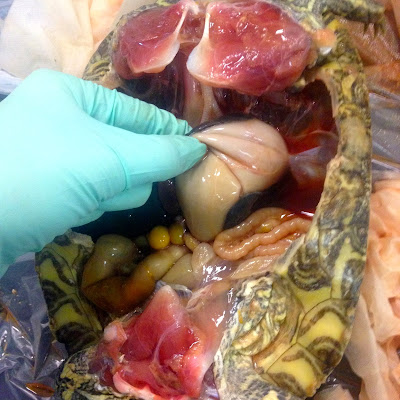
Once you're inside, the most obvious difference in turtle is probably the location of the scapulas, or shoulder blades. Whereas ours are on our back on the dorsal surface, in turtles they are the complete opposite. Their shoulder blades are located where our collar bones are. The rest of the organs are pretty easy to distinguish- trachea, esophagus, stomach, intestines, heart, liver, and so one. When we opened up our turtle and started looking around, the first thing we noticed was an abnormally large liver. We did have to ask about that, because I have no idea what the normal size of a liver in a red eared slider is. The liver also had an abscess on it, which looked like a darker, harder area on the liver. The second thing we noticed was there was a problem with our turtle's heart. Turtles have a three-chambered heart, made up of two atria and one ventricle. (Humans and other mammals have a four-chambered heart made up of two atria and two ventricles.) And our turtle had one atrium that was substantially larger than the other- and this one I knew for sure, because I had something to compare it to. The enlarged atrium also had an abscess on it. You might be able to imagine that that would cause a lot of problems for the poor turtle.
Enlarged liver. You can also see that the scapula are on the ventral surface, where our collar bones would be.
Heart with an atrial abscess. The abscess is the whiter part on the dark red atrium. You can see how much larger it is than the other atrium.
For the first turtle we just finished by doing essentially an exploration or anything else we could see. Turtles and most reptiles don't have a diaphragm, so they don't have a separate thoracic and abdominal cavity the way that we do. Everything is pretty much all lumped in there. In fact, the left lung sac was actually sitting under the stomach. If that happened to a dog, that would be a pretty big problem.
We found the tracheal bifurcation and followed it to each lung sac, which is much different from human and other mammalian lungs. In turtles the lungs are attached to the carapace, or the upper portion of their shell. There is no negative pressure in the lungs, so that if the shell is fractured the turtle can still breathe. Also, as a fun fact, turtles can't cough. It's one of the reasons they are so highly susceptible to pneumonia. We also followed the esophagus to the stomach, which was pretty unremarkable, and then to the mass of intestines. The small intestines were actually pretty cool, because they have this ringed pattern to them, which almost makes them look more like worms.
Pulling out the stomach for better visualization. You can see the small intestines here as well, which look sort of like worms.
Then we moved onto a second turtle, looking for something interesting. The second turtle actually had a shell fracture, right down the middle at the base of the shell, over the tail. She had been paralyzed in the hind limbs, and we wanted to see if she had had either a broken pelvis or some kind of nerve damage. When we opened her up, one big difference jumped out right away, which was that her abdomen was full of eggs. The amount of space dedicated to reproduction in turtles is insane. There were literally eggs everywhere. We opened one up, but all it was inside was a frozen yolk, which was just from freezing the turtle for preservation. Turtle egg shells are surprisingly soft and squishy though. They're like lizard eggs, and this actually really through me off the first time my lizards laid eggs, because I was expecting something much harder. But this turtle also had multiple stages of egg development inside of her, so that was interesting to see. It's always sad doing a necropsy on a pregnant animal, but since she was paralyzed in the hind limbs, I'm sure she would have had a lot of difficultly delivering the eggs, if she even would have been able to.
Female turtle with her eggs. All of the little yellow round ball things are developing eggs.
We tried to get down to her vertebrae to see if there was nerve damage, but we never were able to get deep enough. I think it's a pretty safe guess that that's what the problem was though. The pelvic nerve plexus in a turtle sits right under where her fracture was. We even removed what we think was the fiberglass over her shell fracture to try and get a better look, but we just couldn't see anything. It did make me more interested in wanting to know about turtle shell repair though, so hopefully we still get that lab! There are a couple of different methods for shell repair that I know about, such as metal bridging, a cable tie method (who knew there was such an awesome use for them!), and using fiberglass and epoxy. I wish I knew more about how to actually use those methods, but hopefully I will be able to have a turtle shell repair blog post in the future.
Removing the fiberglass to expose the shell fracture. This shell fracture was perfectly down the middle of the shell.
And just on a random side note, I had noticed that our first turtle had stitches under her tail, so I asked what they were about. And apparently this turtle had been part of a research group for laser therapy. The turtles all received an incision by the tail, and then half of the group received laser therapy while the other half received only conventional medicine. And that study found that laser therapy significantly sped up the healing process. Pretty cool.
Stitches
I love all turtles, and I love my tortoise. And I love learning about them and what makes them so unique and different. =]