It's been a while since my last post... I wanted to have some updates on what clinics were like, but time really got away from me last year in clinics. Needless to say though, clinical year was awesome. The transition from thinking like a student to thinking like a doctor is so subtle that you don't even notice it happening, until you look back and realize how far you've come. And not just in medical knowledge or skills, but in confidence as well. But then you graduate, and suddenly you realize that everything is completely on you. Now you are the only line of defense for a sick animal, and their health is completely dependent on you. It's terrifying. What if you miss something? What if you forget something? How should you decide which drug is best, or what dose to give?
Those questions were plaguing me all summer. I was so excited but also so anxious to start my new job as an associate veterinarian. I kept worrying that I wouldn't be able to remember all the information I'd crammed into my brain the past 4 years. I found myself going over drug doses and side effects, common dermatology problems, and vaccination schedules. In my typical student mindset, studying helped alleviate some of the nerves.
My first day of work is such a blur. I didn't do much of actual doctoring, with all the paperwork and meetings I had to take care of. I was actually disappointed that I didn't get to do more. Then I started seeing some simple appointments- vaccinations, boarding check ins, things that didn't require much of a work up. I helped out the techs with catheter placements, blood draws and anesthesia to refresh my memory of all of those critical skills. And then it felt like I went from one day doing only vaccinations to suddenly having patients. And with each exam, I started feeling more and more confident. Of course I could do this. I survived 8 years of school and 4 years of not having a social life for this. I know how to do this.
Now, I love my job. I wouldn't trade it for anything. I never know what the day is going to bring me. I've seen some of the typical things in dogs and cats- ear infections, cranial cruciate disease, skin problems, and vomiting. I've seen birds for nail trims, beak trims and wing times. I've seen rabbits, guinea pigs, bearded dragons, and ball pythons. I helped with my first every rabbit neuter, and performed my first dog neuter and spay as a licensed veterinarian. I performed my first dental extraction. Once I got going, I was really going. Now I have my rhythm down and I feel unstoppable.
There's still a lot I don't know, and a lot I'm unsure of. Sometimes I feel really overwhelmed, with no idea what to do. But other times I feel like I know exactly what to do, and I always feel so gratified when the client is grateful for my help. It really makes my day when a client tells me how thankful they are for my help. I feel like I'm really doing something right when clients tell me they'll ask for me next time they come in to the clinic. I feel like all of those years I put into getting where I am are starting to pay off, and I've only been working for 2 weeks. I can't wait to see what else the future holds!
Scrubs, Stethoscopes, and Scalpels- The Life of a Vet Student
Sunday, July 12, 2015
Thursday, January 30, 2014
Ophthalmology
I love ophthalmology. Which I never thought I would say. I used to think eyeballs were gross. Now I think they're cool. Maybe this is part of what they were talking about in first year on how passionate and crazy teachers can convert you. Or maybe it's because Sydney's eyes have been horrible for the past 3 years and it's part of my daily routine to stare at and play with eyes now. Either way, I love it.
Today I had my introductory lab to ophthalmology, where we went over all the basic diagnostic tests and got to test them out ourselves. I can't wait until I have my ophthalmology rotation in 2 weeks... 2 afternoons shadowing vets looking at eyeballs is going to be awesome.
Then we get into the fun stuff. We did a Schirmer Tear Test to measure tear production, a fluorescein test to look at the cornea, and we did direct and indirect ophthalmoscopy to look at the back of the eye (fundus). We also got to use tonometry to test the pressure of the eye.
Midori, my green iguana. I love her eyes =]
Jack, my bearded dragon. I love how orange his iris is.
What's cool about these tests too is that some of them can be done at home, if you know how to do them. I've tried them on my lizards, but I don't think the reflexes are the same as in cats and dogs. Two of the tests we did we do so often in veterinary medicine that I forget they're ophthalmic tests... and that would be the menace and palpebral reflex. For the menace response, you cover up one eye on your dog, and then move your hand towards their eyeball, and they should blink. It's like the flinch response that people have. And then in one test, you've already tested two nerves- the optic nerve (the ability to see) and the facial nerve (the ability to blink). For the palpebral reflex, you gently tap at the corner or the eye, and the dog should blink. We use this all the time in anesthesia to make sure the patient is deep enough, because if they are properly sedated they won't have the palpebral reflex, but they will if they're light. And again, this is testing two nerves- the trigeminal nerve (sensation on the face) and the facial nerve (the ability to blink). Another ophthalmic test is another one people can do, and that we do do on a regular basis- tracking. As in, can my dog see this object and follow it. In clinics we use cotton balls, because we know that the dog isn't smelling it or hearing it, and if they follow it they definitely have to see it. And all you have to do is wave it around, drop it on the ground, ect. and watch the dog's eyes or head follow it. And with that, you know the dog can see, and if their eyeballs moved, you've tested the oculomotor nerve and abducens nerve, both of which control eyeball movement.
Then we get into the fun stuff. We did a Schirmer Tear Test to measure tear production, a fluorescein test to look at the cornea, and we did direct and indirect ophthalmoscopy to look at the back of the eye (fundus). We also got to use tonometry to test the pressure of the eye.
For the Schirmer Tear Test, you take a test strip and stick it in the eye, essentially causing irritation to cause tear production. And it does pretty much what the name implies- it's a tear test, so we want to know that a dog can produce adequate tear film. Tear film is what provides nutrition to the cornea, and if you don't have enough there are a whole bunch of problems that go along with that. The test strip has a line of blue dye that wicks up with the tears so you can read it. Normal production in a dog is over 15.
With the fluorescein stain we are looking to see if there is an ulceration in the cornea. The fluorescein dye is this bright, neon green dye that sticks to the stroma of the cornea, which is the layer under the epithelial (top) layer. If there is a corneal ulcer, the epithelium is usually gone, and so the dye sticks to the exposed stroma. So you just have to take a drop and place it in the eye, and then you shine your light into the eyeball, and if there is any fluorescein uptake you will see bright, neon green. If you don't see anything, there is no stromal exposure and probably no ulcer. Which is good.
Sydney after her fluorescein stain- you can still see all the bright green dye around her eye. But no ulcers, yay!
Direct ophthalmoscopy is what they do to you at the doctor's office, where they get that instrument and shine a light right in your eyes. Animal eyes are so much cooler than primate eyes though. Using this we can see the optic nerve and all of the blood vessels in the fundus of the eye. Indirect ophthalmoscopy provides the same end result, but you can look at the whole fundus at once, whereas with direct it is much more magnified and you can only see a small area at a time. That can be fun, because you can look around and find the optic nerve, and then the dog moves it's eyes or head and you get to start over. You don't have that problem with the indirect method, because you're able to see the entire fundus at once.
What you would see with indirect ophthalmology... the little circle in the center is where the optic nerve comes in, known as the optic disc. All the little red squiggly lines are blood vessels. The darker part is the choroid, and the top, lighter part is the tapetum. The tapetum is what makes some animals' eyes seem to glow when you shine a light in them ("eye shine")
Tonometry is my favorite test, just because I think it's cool. Tonometry is a test to measure pressure in the eye, which is most important with glaucoma. We have two different ways to measure pressure. One method uses a little round pin, which shoots out and bounces off of the cornea. The instrument measures the speed with which it returned and gives you a reading based off of that. The other method uses a tonopen, where you press directly on the cornea. You need topical anesthetic in the eye for this one, but then you just push the little probe onto the cornea, and you see the cornea indent a little bit, and you just keep doing that until it beeps at you and you get your reading. Normal pressure in a dog is between 10-20. With glaucoma, the readings are much much higher, like in the 40's and 50's. I really want a tonopen because they're small enough that you can use them on exotics. The only problem is they're like $3000... but since my lizard has such horrible eye problems, I think it could be a good investment in the future. But first I'd have to find out what normal pressure in a lizard eye is.
There is a whole lot more with eyeballs that I could get into, but I won't. At least, not until I have my ophthalmology rotation.
A dissected eyeball
And just for fun...
An albino python eye
A crocodile eye
A stingray eye
And if you want to see more cool animal eyes, check out this website Nature's Ocular DiversityTuesday, January 14, 2014
Final Semester!
It's been a while since I've updated this blog. Things were so crazy at the end of last semester that I actually had a post saved as a draft and completely forgot to publish it. And it seemed a little pointless to publish it now. But I survived another semester with about three surgeries a week, and I am one step closer to being done!
This is my last semester of actual coursework. It feels so weird saying that, and I can't believe that in May I'm going to be entering clinical rotations. I don't know what I'm going to do with myself. After the hectic schedule I had last semester, I'm excited to take it a little easier this semester. The best part is I'm done surgery! I completed my three assistant surgeon days, my three primary surgeon days, and seven days as the anesthetist. And then I survived my anesthesia grilling, which is where a surgeon goes over all the aspects of anesthesia with you to make sure you learned something, and you sit there and feel really smart for about 25% of the time and like a complete idiot for the other 75% of the time. But that's all over! I kind of miss my primary surgeon rotations, but not enough to go back. Since I didn't update anything on how they went, I'll just say briefly that they went really well, all six of my puppies are doing good, and it was probably one of my biggest accomplishments in school to date. But it was also a lot of work and I always left exhausted, so I don't mind having a break this semester.
This semester I also have my second introduction to clinics rotation, where twice a week we go to the hospital and shadow senior students on their rotations. I'm very excited to get into the clinics and start putting all this knowledge I've had crammed into my head to use! On the other hand, I'm a nervous wreck thinking about starting clinics. Right now I feel completely unprepared, so I'm really hoping that this clinics course changes that.
But even more that clinics at the hospital, I am beyond excited for my externships! I absolutely loved working in South Africa, and it pretty much solidified for me that I want to work in exotic animal medicine. Even though we did a lot of repetitive work, especially on our sable days where we did the same thing for about 14 sable per day for three days, it was the first time I didn't get bored doing the same thing. I lasted about 2 days in a small animal clinic before I started feeling claustrophobic and had to get out. I made it longer in equine medicine, but I was still getting bored. And it's crazy, because when I started vet school I was so sure I was going to practice in equine medicine, and any time a professor mentioned how some students always end up doing the complete opposite of what they saw themselves doing in the beginning of vet school, I always thought that will never be me. And now it is! I can't imagine doing any other work now. I love how each day you're doing something different with exotics, and how you never know what the day will bring. And I love feeling like I can make a real difference to conservation at the same time.
So following this, I knew I wanted my externships to be in the exotics field, preferably zoo medicine. The problem is it is super competitive, and they only take one student at a time. But I managed to get a 6 week externship at both the Philadelphia Zoo and the Oklahoma City Zoo! I can't wait for the experiences I'm going to get with 3 months in zoo medicine. It's going to be unreal. But as excited as I am for them, I also don't want to rush through my last semester sitting in a classroom. But only because I don't think I'm ready to end up in the real world yet!
This is my last semester of actual coursework. It feels so weird saying that, and I can't believe that in May I'm going to be entering clinical rotations. I don't know what I'm going to do with myself. After the hectic schedule I had last semester, I'm excited to take it a little easier this semester. The best part is I'm done surgery! I completed my three assistant surgeon days, my three primary surgeon days, and seven days as the anesthetist. And then I survived my anesthesia grilling, which is where a surgeon goes over all the aspects of anesthesia with you to make sure you learned something, and you sit there and feel really smart for about 25% of the time and like a complete idiot for the other 75% of the time. But that's all over! I kind of miss my primary surgeon rotations, but not enough to go back. Since I didn't update anything on how they went, I'll just say briefly that they went really well, all six of my puppies are doing good, and it was probably one of my biggest accomplishments in school to date. But it was also a lot of work and I always left exhausted, so I don't mind having a break this semester.
This semester I also have my second introduction to clinics rotation, where twice a week we go to the hospital and shadow senior students on their rotations. I'm very excited to get into the clinics and start putting all this knowledge I've had crammed into my head to use! On the other hand, I'm a nervous wreck thinking about starting clinics. Right now I feel completely unprepared, so I'm really hoping that this clinics course changes that.
But even more that clinics at the hospital, I am beyond excited for my externships! I absolutely loved working in South Africa, and it pretty much solidified for me that I want to work in exotic animal medicine. Even though we did a lot of repetitive work, especially on our sable days where we did the same thing for about 14 sable per day for three days, it was the first time I didn't get bored doing the same thing. I lasted about 2 days in a small animal clinic before I started feeling claustrophobic and had to get out. I made it longer in equine medicine, but I was still getting bored. And it's crazy, because when I started vet school I was so sure I was going to practice in equine medicine, and any time a professor mentioned how some students always end up doing the complete opposite of what they saw themselves doing in the beginning of vet school, I always thought that will never be me. And now it is! I can't imagine doing any other work now. I love how each day you're doing something different with exotics, and how you never know what the day will bring. And I love feeling like I can make a real difference to conservation at the same time.
So following this, I knew I wanted my externships to be in the exotics field, preferably zoo medicine. The problem is it is super competitive, and they only take one student at a time. But I managed to get a 6 week externship at both the Philadelphia Zoo and the Oklahoma City Zoo! I can't wait for the experiences I'm going to get with 3 months in zoo medicine. It's going to be unreal. But as excited as I am for them, I also don't want to rush through my last semester sitting in a classroom. But only because I don't think I'm ready to end up in the real world yet!
Thursday, November 7, 2013
Turtle Necropsy
A lot of people seemed to be really interested in my previous reptile necropsy post. And hey, I was pretty interested in it too. I was able to have a similar experience recently, but this time we only necropsied red eared sliders. We were supposed to be doing a shell repair lab, but unfortunately we ran out of time for that one. Hopefully sometime in the future I'll be able to actually work on that.
So as I mention, we necropsied red eared sliders for this wet lab. Most of the sliders had been part of a research group, and the cause of death was unknown. That wasn't really why we were originally intending to do this, but that's what we ended up doing.
The major difference between necropsying a turtle and any other reptile is that really hard thing called a shell that gets in your way. We had to saw through the shells on all of the turtles to gain access to the rest of the body cavities. And the muscles on a turtle's legs are actually attached to the shell on the inside, so you can't just saw one side open and then lift it up like your opening a lid. It takes a surprising amount of work. In fact, it took us longer to get the shell off than it did to identify the cause of death.
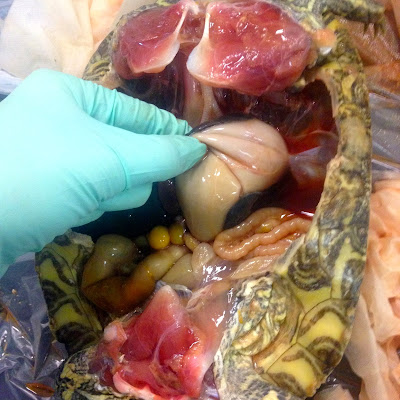
Once you're inside, the most obvious difference in turtle is probably the location of the scapulas, or shoulder blades. Whereas ours are on our back on the dorsal surface, in turtles they are the complete opposite. Their shoulder blades are located where our collar bones are. The rest of the organs are pretty easy to distinguish- trachea, esophagus, stomach, intestines, heart, liver, and so one. When we opened up our turtle and started looking around, the first thing we noticed was an abnormally large liver. We did have to ask about that, because I have no idea what the normal size of a liver in a red eared slider is. The liver also had an abscess on it, which looked like a darker, harder area on the liver. The second thing we noticed was there was a problem with our turtle's heart. Turtles have a three-chambered heart, made up of two atria and one ventricle. (Humans and other mammals have a four-chambered heart made up of two atria and two ventricles.) And our turtle had one atrium that was substantially larger than the other- and this one I knew for sure, because I had something to compare it to. The enlarged atrium also had an abscess on it. You might be able to imagine that that would cause a lot of problems for the poor turtle.
So as I mention, we necropsied red eared sliders for this wet lab. Most of the sliders had been part of a research group, and the cause of death was unknown. That wasn't really why we were originally intending to do this, but that's what we ended up doing.
The major difference between necropsying a turtle and any other reptile is that really hard thing called a shell that gets in your way. We had to saw through the shells on all of the turtles to gain access to the rest of the body cavities. And the muscles on a turtle's legs are actually attached to the shell on the inside, so you can't just saw one side open and then lift it up like your opening a lid. It takes a surprising amount of work. In fact, it took us longer to get the shell off than it did to identify the cause of death.
Opening the shell
Once you're inside, the most obvious difference in turtle is probably the location of the scapulas, or shoulder blades. Whereas ours are on our back on the dorsal surface, in turtles they are the complete opposite. Their shoulder blades are located where our collar bones are. The rest of the organs are pretty easy to distinguish- trachea, esophagus, stomach, intestines, heart, liver, and so one. When we opened up our turtle and started looking around, the first thing we noticed was an abnormally large liver. We did have to ask about that, because I have no idea what the normal size of a liver in a red eared slider is. The liver also had an abscess on it, which looked like a darker, harder area on the liver. The second thing we noticed was there was a problem with our turtle's heart. Turtles have a three-chambered heart, made up of two atria and one ventricle. (Humans and other mammals have a four-chambered heart made up of two atria and two ventricles.) And our turtle had one atrium that was substantially larger than the other- and this one I knew for sure, because I had something to compare it to. The enlarged atrium also had an abscess on it. You might be able to imagine that that would cause a lot of problems for the poor turtle.
Enlarged liver. You can also see that the scapula are on the ventral surface, where our collar bones would be.
Heart with an atrial abscess. The abscess is the whiter part on the dark red atrium. You can see how much larger it is than the other atrium.
For the first turtle we just finished by doing essentially an exploration or anything else we could see. Turtles and most reptiles don't have a diaphragm, so they don't have a separate thoracic and abdominal cavity the way that we do. Everything is pretty much all lumped in there. In fact, the left lung sac was actually sitting under the stomach. If that happened to a dog, that would be a pretty big problem.
We found the tracheal bifurcation and followed it to each lung sac, which is much different from human and other mammalian lungs. In turtles the lungs are attached to the carapace, or the upper portion of their shell. There is no negative pressure in the lungs, so that if the shell is fractured the turtle can still breathe. Also, as a fun fact, turtles can't cough. It's one of the reasons they are so highly susceptible to pneumonia. We also followed the esophagus to the stomach, which was pretty unremarkable, and then to the mass of intestines. The small intestines were actually pretty cool, because they have this ringed pattern to them, which almost makes them look more like worms.
Pulling out the stomach for better visualization. You can see the small intestines here as well, which look sort of like worms.
Then we moved onto a second turtle, looking for something interesting. The second turtle actually had a shell fracture, right down the middle at the base of the shell, over the tail. She had been paralyzed in the hind limbs, and we wanted to see if she had had either a broken pelvis or some kind of nerve damage. When we opened her up, one big difference jumped out right away, which was that her abdomen was full of eggs. The amount of space dedicated to reproduction in turtles is insane. There were literally eggs everywhere. We opened one up, but all it was inside was a frozen yolk, which was just from freezing the turtle for preservation. Turtle egg shells are surprisingly soft and squishy though. They're like lizard eggs, and this actually really through me off the first time my lizards laid eggs, because I was expecting something much harder. But this turtle also had multiple stages of egg development inside of her, so that was interesting to see. It's always sad doing a necropsy on a pregnant animal, but since she was paralyzed in the hind limbs, I'm sure she would have had a lot of difficultly delivering the eggs, if she even would have been able to.
Female turtle with her eggs. All of the little yellow round ball things are developing eggs.
We tried to get down to her vertebrae to see if there was nerve damage, but we never were able to get deep enough. I think it's a pretty safe guess that that's what the problem was though. The pelvic nerve plexus in a turtle sits right under where her fracture was. We even removed what we think was the fiberglass over her shell fracture to try and get a better look, but we just couldn't see anything. It did make me more interested in wanting to know about turtle shell repair though, so hopefully we still get that lab! There are a couple of different methods for shell repair that I know about, such as metal bridging, a cable tie method (who knew there was such an awesome use for them!), and using fiberglass and epoxy. I wish I knew more about how to actually use those methods, but hopefully I will be able to have a turtle shell repair blog post in the future.
Removing the fiberglass to expose the shell fracture. This shell fracture was perfectly down the middle of the shell.
And just on a random side note, I had noticed that our first turtle had stitches under her tail, so I asked what they were about. And apparently this turtle had been part of a research group for laser therapy. The turtles all received an incision by the tail, and then half of the group received laser therapy while the other half received only conventional medicine. And that study found that laser therapy significantly sped up the healing process. Pretty cool.
Stitches
I love all turtles, and I love my tortoise. And I love learning about them and what makes them so unique and different. =]
Tuesday, November 5, 2013
Assistant Surgeon
Part of our junior surgery class is participating in live spay and neuter surgeries, usually working under 4th year students who are currently taking the shelter medicine rotation. We have to do about 12 surgeries total; 6 as an anesthetist, 3 as an assistant surgeon, and 3 as the primary surgeon. I'm not going to lie, I was pretty nervous. I had my surgeries scheduled before I even finished my cadaver surgeries, and I was not at all confident that one spay would prepare me to be an assistant surgeon. I couldn't even do my neuter, because my dog came already neutered. Not the best situation help with confidence levels.
But I have now completed all three of my assistant surgeon roles in live surgery. And while I had something pretty close to a panic attack before my first one, looking back I really enjoyed them. I learned way more from the live surgeries than I ever could have from the cadaver labs, and my confidence shot way up. Usually my 4th year primary surgeon let me do about a third of the procedure, such as removing one testis, or tying the Miller's Knot around the uterine body and transecting the uterine body. I didn't even learn the Miller's Knot until the live surgeries, even though that's all they use. I also learned how to do an instrument tie, although I'm not quite as proficient at that yet. I'll need to practice that.
With every surgery, I always feel like the beginning is just a huge mass of confusion. The anesthetists are trying to figure out what instruments need to be gathered for the catheter and endotracheal tube placement, the assistant surgeons are gathering gowns and surgery packs and gloves for the actual surgery, and I have no idea what the primary surgeons are getting ready. The beginning always feels like no one knows exactly what we should be doing. But then once we move to actually operate, I feel like everything always calms down. Anesthesia is all hooked up and only has to monitor, and the surgeons just kind of do their thing. And occasionally there is some excitement if the anesthesia gets a little light- usually it took took too long to actually begin the surgery and the anesthetic shot we give in induction is wearing off- but that has been the only wrench I've encountered so far. Knock on wood.
For the assistant surgeon role, the main job is obviously to assist the primary surgeon. This means that I would be responsible for doing the physical exams on the patients first, to make sure they are actually healthy enough for surgery. Then some of the patients get different shots with us too- some get rabies, some get distemper, some even get microchipped. I get to do all of that, and record where they shots were give to watch for an injection site reaction. Then after the patient has been induced and intubated by anesthesia, I prep the surgery site. So I clip the hair and perform a primary sterile scrub with chlorhexidine. Once the patient has been moved onto the operating table, I do a secondary sterile scrub. Then I go scrub in while the primary surgeon drapes the patient.
During the surgery, the only guidelines are to do whatever the primary surgeon asks for. Usually this involves handing over instruments, holding things out of the way such as mosquito clamps holding tissue, or helping keep suture tight. As I mentioned earlier, if the surgery is going well and appears to be uncomplicated, I have also been able to perform actual pieces of the surgery. On my second surgery as assistant surgeon I exteriorized a testis, stripped down all the spermatic fascia, clamped, sutured, and cut the spermatic cord. I learned so much more from doing this one testis than I did from anything in the cadaver lab. It may seem obvious, but dead animals don't bleed, and live animals bleed a lot. So you don't get that experience of looking and checking for hemorrhage and looking for structures through blood until you actually do a live surgery. The experience is really invaluable.
Then after the surgery, the people responsible for anesthesia stay with the patients until they wake up. You have to watch for the first attempt to swallow, and that is when it is safe to remove the endotracheal tube, but you always have to check for reflux to make sure nothing goes back down to the lungs that shouldn't. If the patients don't recover in time (our animals are all from local shelters, so they are picked up to be taken back to the shelters at a specific time) it is the assistant surgeon that stays with them and is responsible for coming in that night and the next morning to check on them and write the SOAP. The SOAP is basically just how the dog is doing- you have your subjective and objective observations, your assessment, and your plant of action. If the dog appears painful on a check up we take them up to ICU for some pain meds. Luckily, since spays and neuters are elective surgeries (for the most part), the animals are usually in pretty good health and there aren't any serious complications. I had one dog that was slow to wake up and her body temperature just would not come up after surgery. She ended up staying the night, but she wasn't painful and while her temperature was still lower than average the next morning, it was higher than it had been the night before and her attitude and demeanor was much better and much more lively. So there is a lot more that matters than just textbook ranges.
Now that I'm done with my assistant surgeries, I am moving on the my three surgeries as the primary surgeon over the next two weeks. I'm not as nervous as I was for my first surgery as the assistant surgeon, but I'm still a little nervous to have all of that responsibility on me. At least now though I feel like I have a much better idea of what to do and how to do it. I just need to practice my interdermal suture closure before then.
But I have now completed all three of my assistant surgeon roles in live surgery. And while I had something pretty close to a panic attack before my first one, looking back I really enjoyed them. I learned way more from the live surgeries than I ever could have from the cadaver labs, and my confidence shot way up. Usually my 4th year primary surgeon let me do about a third of the procedure, such as removing one testis, or tying the Miller's Knot around the uterine body and transecting the uterine body. I didn't even learn the Miller's Knot until the live surgeries, even though that's all they use. I also learned how to do an instrument tie, although I'm not quite as proficient at that yet. I'll need to practice that.
With every surgery, I always feel like the beginning is just a huge mass of confusion. The anesthetists are trying to figure out what instruments need to be gathered for the catheter and endotracheal tube placement, the assistant surgeons are gathering gowns and surgery packs and gloves for the actual surgery, and I have no idea what the primary surgeons are getting ready. The beginning always feels like no one knows exactly what we should be doing. But then once we move to actually operate, I feel like everything always calms down. Anesthesia is all hooked up and only has to monitor, and the surgeons just kind of do their thing. And occasionally there is some excitement if the anesthesia gets a little light- usually it took took too long to actually begin the surgery and the anesthetic shot we give in induction is wearing off- but that has been the only wrench I've encountered so far. Knock on wood.
For the assistant surgeon role, the main job is obviously to assist the primary surgeon. This means that I would be responsible for doing the physical exams on the patients first, to make sure they are actually healthy enough for surgery. Then some of the patients get different shots with us too- some get rabies, some get distemper, some even get microchipped. I get to do all of that, and record where they shots were give to watch for an injection site reaction. Then after the patient has been induced and intubated by anesthesia, I prep the surgery site. So I clip the hair and perform a primary sterile scrub with chlorhexidine. Once the patient has been moved onto the operating table, I do a secondary sterile scrub. Then I go scrub in while the primary surgeon drapes the patient.
During the surgery, the only guidelines are to do whatever the primary surgeon asks for. Usually this involves handing over instruments, holding things out of the way such as mosquito clamps holding tissue, or helping keep suture tight. As I mentioned earlier, if the surgery is going well and appears to be uncomplicated, I have also been able to perform actual pieces of the surgery. On my second surgery as assistant surgeon I exteriorized a testis, stripped down all the spermatic fascia, clamped, sutured, and cut the spermatic cord. I learned so much more from doing this one testis than I did from anything in the cadaver lab. It may seem obvious, but dead animals don't bleed, and live animals bleed a lot. So you don't get that experience of looking and checking for hemorrhage and looking for structures through blood until you actually do a live surgery. The experience is really invaluable.
Then after the surgery, the people responsible for anesthesia stay with the patients until they wake up. You have to watch for the first attempt to swallow, and that is when it is safe to remove the endotracheal tube, but you always have to check for reflux to make sure nothing goes back down to the lungs that shouldn't. If the patients don't recover in time (our animals are all from local shelters, so they are picked up to be taken back to the shelters at a specific time) it is the assistant surgeon that stays with them and is responsible for coming in that night and the next morning to check on them and write the SOAP. The SOAP is basically just how the dog is doing- you have your subjective and objective observations, your assessment, and your plant of action. If the dog appears painful on a check up we take them up to ICU for some pain meds. Luckily, since spays and neuters are elective surgeries (for the most part), the animals are usually in pretty good health and there aren't any serious complications. I had one dog that was slow to wake up and her body temperature just would not come up after surgery. She ended up staying the night, but she wasn't painful and while her temperature was still lower than average the next morning, it was higher than it had been the night before and her attitude and demeanor was much better and much more lively. So there is a lot more that matters than just textbook ranges.
Now that I'm done with my assistant surgeries, I am moving on the my three surgeries as the primary surgeon over the next two weeks. I'm not as nervous as I was for my first surgery as the assistant surgeon, but I'm still a little nervous to have all of that responsibility on me. At least now though I feel like I have a much better idea of what to do and how to do it. I just need to practice my interdermal suture closure before then.
Sunday, September 22, 2013
Happy World Rhino Day!!
Today is World Rhino Day!
It's the perfect time to stop and appreciate a magnificent species and to aid the conservation effort =]
My husband and I are both becoming more actively involved with Rhino Rescue Project, the group that we worked with in South Africa to perform the rhino horn infusions. We are both passionate about helping to save this species and are happy to help in any way that we can.
My husband did this amazing art piece for Rhino Rescue Project in honor of World Rhino Day. It's a continuation of his new word art series, and all of the words that make up the picture are related to rhino conservation.
![]()
It's the perfect time to stop and appreciate a magnificent species and to aid the conservation effort =]
My husband and I are both becoming more actively involved with Rhino Rescue Project, the group that we worked with in South Africa to perform the rhino horn infusions. We are both passionate about helping to save this species and are happy to help in any way that we can.
My husband did this amazing art piece for Rhino Rescue Project in honor of World Rhino Day. It's a continuation of his new word art series, and all of the words that make up the picture are related to rhino conservation.
I've been working on a new sewing project, where I am hand embroidering rhinos and am planning to make pillows and wall hangings out of them. I am hoping to be able to sell them and give a portion of the proceeds to Rhino Rescue Project.
And since we were so successful with our bake sale fundraising last year, we are hoping to do our mobile bake sale again, with cupcakes and safari shaped cookies! Not only is baking a great way to procrastinate from studying, but then by selling them I don't have to feel guilty and obliged to eat everything I bake, and I can fundraise for rhinos at the same time! It's a win-win situation. =]
And hopefully one day we'll be able to go back to South Africa and work with Rhino Rescue Project again.
If you're interested in checking out the awesome work that Rhino Rescue Project is doing, here's the link to their facebook. Rhino Rescue Project
Tuesday, September 17, 2013
Just another day in the VMTH
It was another exciting equine med class today, as we got to try our hand at passing nasogastric tubes and performing abdominocentesis. All of this was complicated by the fact that the horses at the hospital have had tubes passed on them so many times that they are nearly impossible to work with. The bright side to that is just if you can get it done on them, you can get it done on any horse! We also had a little help... I gave our horse some xylazine IV to help calm her down. But even with that she still put up a fight.
I'd never passed a nasogastric tube before, though I'd seen it done. There are a lot of reasons to tube a horse, but the most common is probably colic. Every horses that is colicking gets a tube. So if you're going to be an equine practitioner, it's another skill you should probably keep in your back pocket, since nearly anything can make a horse colic.
The trick- and scary part, if you've never done it before- is actually passing the tube into the esophagus and not the trachea. It's surprising easy for the tube to go the wrong way, since it just naturally wants to go dorsal and not ventral. So once you get the horse to swallow and you think you have the tube in the right spot, you always have to stop and check. You should be able to feel two tubes in the horse's neck- one tube is the trachea, and the other tube is the nasogastric tube in the esophagus. When you suck in on the other end of the tube as well, there should be negative pressure (aka no air). Once you've confirmed you're in the trachea, you just keep sliding the tube down, blowing into it as you go to help expand the esophagus, until you stop getting negative pressure and you start getting positive pressure (aka air) when you suck in. Sometimes the change is very abrupt, and you end up inhaling stomach acid and pulling a bunch of stomach contents back up the tube. But at least you know you're in the right spot then! And sometimes exactly what you want is stomach contents, anyway.
For abdominocentesis, you have to feel for the xiphoid, or the end of the sternum. There are two muscles on the horse's belly that form a V right at the xiphoid. From there you go out a hand's width and a little to the right (to avoid the spleen that is sitting on the left), and just stick a needle in there. Nothing should happen with a normal horse, but with a sick horse you might get fluid coming out. Abdominocentesis isn't done a lot in equine medicine- you have to weigh the risks and benefits to a procedure, and our horse was a kicker- but it's also done for colics. If a horse is colicking and you get clear fluid, that's a better prognostic sign than if you get blood and a whole bunch of other nasty fluid, which might indicated a perforated bowel.
![]()
I'd never passed a nasogastric tube before, though I'd seen it done. There are a lot of reasons to tube a horse, but the most common is probably colic. Every horses that is colicking gets a tube. So if you're going to be an equine practitioner, it's another skill you should probably keep in your back pocket, since nearly anything can make a horse colic.
The trick- and scary part, if you've never done it before- is actually passing the tube into the esophagus and not the trachea. It's surprising easy for the tube to go the wrong way, since it just naturally wants to go dorsal and not ventral. So once you get the horse to swallow and you think you have the tube in the right spot, you always have to stop and check. You should be able to feel two tubes in the horse's neck- one tube is the trachea, and the other tube is the nasogastric tube in the esophagus. When you suck in on the other end of the tube as well, there should be negative pressure (aka no air). Once you've confirmed you're in the trachea, you just keep sliding the tube down, blowing into it as you go to help expand the esophagus, until you stop getting negative pressure and you start getting positive pressure (aka air) when you suck in. Sometimes the change is very abrupt, and you end up inhaling stomach acid and pulling a bunch of stomach contents back up the tube. But at least you know you're in the right spot then! And sometimes exactly what you want is stomach contents, anyway.
For abdominocentesis, you have to feel for the xiphoid, or the end of the sternum. There are two muscles on the horse's belly that form a V right at the xiphoid. From there you go out a hand's width and a little to the right (to avoid the spleen that is sitting on the left), and just stick a needle in there. Nothing should happen with a normal horse, but with a sick horse you might get fluid coming out. Abdominocentesis isn't done a lot in equine medicine- you have to weigh the risks and benefits to a procedure, and our horse was a kicker- but it's also done for colics. If a horse is colicking and you get clear fluid, that's a better prognostic sign than if you get blood and a whole bunch of other nasty fluid, which might indicated a perforated bowel.
Getting the nasogastric tube into the ventral medial meatus is the most difficult part
sliding the tube down into the esophagus
checking for negative pressure
Subscribe to:
Comments (Atom)